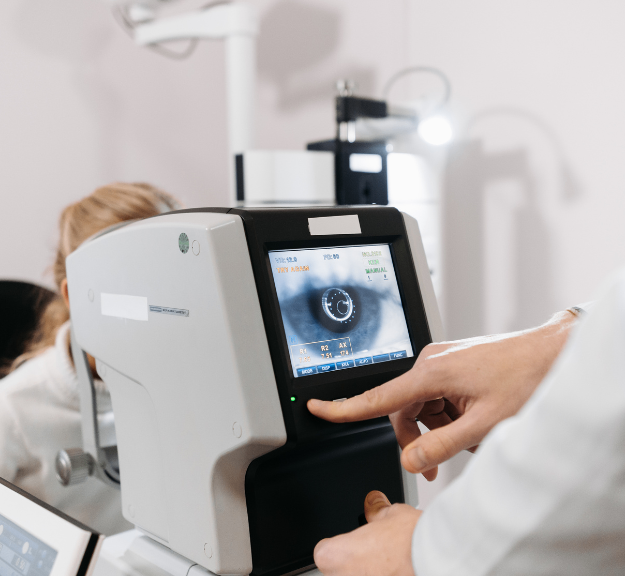
Regulatory Affairs professionals ensure medical devices meet global compliance standards and navigate complex regulatory frameworks like MDR and IVDR. They play a crucial role in securing product approvals and maintaining ongoing compliance to ensure patient safety.

From life-changing diagnostics to next-gen wearable tech, the medical devices industry is advancing healthcare at a rapid pace. We connect pioneering MedTech businesses with exceptional talent across the full product lifecycle -from R&D and regulatory affairs to commercialisation.

ROLES WE PLACE IN THIS SPACE
- Regulatory Affairs Manager
- Quality Assurance Specialist
- Clinical Affairs Associate
- R&D Project Manager
- Medical Device Sales Director
Specialists in Medical Device Recruitment
Whether you're scaling a start-up with breakthrough diagnostics or expanding a global MedTech brand, we understand the nuances of medical device hiring. Our team of recruiters are experts in this space, bringing market knowledge and a tailored, consultative approach to every project.
Quality Assurance experts focus on maintaining the highest standards in product design, manufacturing, and post-market monitoring. Their work ensures that devices consistently meet stringent industry regulations and deliver reliable performance.
This discipline covers the entire lifecycle of medical device creation, from concept and prototyping to testing and market launch. Engineers and designers collaborate to develop innovative, user-friendly devices that meet clinical and patient needs.
Professionals in Clinical and Medical Affairs bridge the gap between product development and healthcare practice. They manage clinical trials, gather evidence of device efficacy, and support the safe and effective use of medical technologies.
Manufacturing and Supply Chain specialists streamline production processes, ensuring devices are produced efficiently and delivered on time. They manage procurement, logistics, and supplier relationships while maintaining compliance with industry standards.
Research and Development teams explore cutting-edge technologies, including artificial intelligence and machine learning, to create smarter, more efficient medical devices. Their work drives innovation, enabling breakthroughs in diagnostics, treatment, and patient monitoring.
We work closely with clients to identify not just technical ability, but the adaptability and compliance awareness critical to success in regulated environments. With an extensive international network, we place permanent and contract professionals across the Europe.
Artificial intelligence and machine learning are revolutionising how medical devices diagnose and monitor health conditions. These technologies enable faster, more accurate data analysis, improving early detection, personalised treatment, and patient outcomes.
As consumers seek more personalised healthcare solutions, wearable devices are becoming essential tools for real-time health tracking. Innovations in biosensors, smartwatches, and remote monitoring systems are reshaping how patients manage their health.
Stricter regulations, such as Europe’s MDR and IVDR, are redefining how medical devices are developed, tested, and approved. Companies must adapt by investing in regulatory expertise to ensure compliance and maintain market access in a highly competitive landscape.

Looking for your next role in MedTech?
Your next career move starts here. Whether you're looking for your next challenge or a step up in your career, we’re here to help you make it happen.
Explore our latest opportunities today:
The latest MedTech trends & innovations
The MedTech industry is experiencing rapid transformation, driven by new technologies, stricter regulations, and evolving patient needs. Businesses must adapt quickly, and that starts with hiring talent that’s ahead of the curve.
AI-powered diagnostics and next-gen wearables are transforming how health data is collected and analysed. This innovation is creating demand for talent skilled in digital health, data science, and device integration.
Stricter MDR and IVDR requirements are reshaping how MedTech products are developed and brought to market. Regulatory, clinical, and quality professionals with up-to-date knowledge are now essential.
With the rise of remote care, there’s growing need for experts in connected devices, telehealth, and user-focused design to develop solutions that improve outcomes beyond the clinic.
Mergers, acquisitions, and global expansion are driving rapid team changes. Companies need agile talent strategies to scale, restructure, and stay competitive across borders.

Testimonials
Trusted by Leading MedTech Companies

Working with Panda has been a great experience from the start. Even in a highly competitive Dutch market, they continue to surprise us by delivering well-matched, high-quality candidates at speed. Their communication is always open and honest, even when challenges arise, which builds real trust.
What stands out is that they go far beyond CV matching. They understand our business, support with visa processes, and offer practical solutions when things get complex. It feels like a true partnership—collaborative, reliable, and focused on outcomes that matter to us.
Within the biopharmaceutical contract industry, you need to act fast. Panda was able to deliver a shortlist of good candidates fast.The interactions with the Panda consultants was very good. We are very pleased with their support and professionality and certainly will consider them for future assignments.
Our HR initially set up the relationship after we had difficulty filling a Quality Engineer position in my team. The relationship with Panda was excellent from the beginning.What I like most about Panda and their consultants, more than other companies, is that they actually listen to my feedback and improve results upon it.I really notice that other parties keep sending candidates that are available, but not necessarily the ones that are the best match. Again, the consultants at Panda are very good listeners and adjusts their course accordingly, but they are also not afraid to let me know what is realistic or not. I would strongly recommend Panda.